Ionic vs Covalent Substance Lab
advertisement

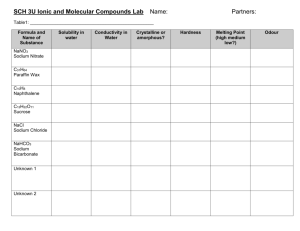
Ionic vs Covalent Substance Lab November 18, 2014 Scenario: You are going to get 4 different substances and you need to be able to uncover which ones contain covalent bonds and which ones contain ionic bonds. Problem Question: Which of the substances contains covalent bonds and which ones contain ionic bonds. Hypothesis: Write a PREDICTION that explains what substance will show which bond type each contains. You will need to have a separate hypothesis for each substance. Substance 1 –Vegetable Oil Substance 2 – Salt Substance 3 – Sugar Substance 4 – Baking Soda Materials Substance 1 –Vegetable Oil Substance 2 – Salt Substance 3 – Sugar Substance 4 – Baking Soda 400 mL beaker 4 test tubes Hot plate Spoon Stop Watch 100 mL graduated cylinder Room Temperature tap water Goggles Procedure Melting Point 1. Begin by gathering 4 test tubes, a 400 mL beaker and a hot plate. 2. Place the substances in each of the designated test tubes. (ex. Substance 1 in test tube #1) 3. Turn the Hot Plate on to high. 4. Place all 4 test tubes into the beaker and place the beaker on the hot plate. 5. Watch and record when each of the substances melts. Procedure Dissolving in Water 1. Begin by gathering a spoon, a 100 mL graduated cylinder and a 400 mL beaker. 2. In the beaker, have the teacher pour in substance #1. 3. Fill the graduated cylinder up to 100 mL with tap water and then pour it into the beaker. 4. Begin stirring the solution in the beaker for 30 seconds and then let it sit for 30 seconds. Record if it dissolves or not. 5. Repeat steps 1 – 4 with the remaining substances. Ionic and Covalent Properties Table Ionic Compounds Covalent Compounds Property: Melting point High Melting Point Low Melting Point Test: Apply heat Result: Won’t melt at low heat Result: Will melt at low heat Property: Dissolve in water Will dissolve in water Will not dissolve in water Test: Put in water Result: Will “disappear” in water Result: will NOT “disappear” in water Property: Crystal formation Test: ?? Will form crystals Usually do not form crystals. Property: Conduct electricity Test: ?? Will conduct electricity IF melted or Will never conduct electricity dissolved in water Data Table Substance Substance #1 Vegetable Oil Substance #2 Salt Substance #3 Sugar Substance #4 Baking Soda Melting Point (High or Low) Dissolves in Water (Yes or No) Get Materials and Begin Working Answers Substance Substance #1 Vegetable oil Substance #2 Salt Substance #3 Sugar Substance #4 Baking Soda Melting Point (High or Low) Dissolves in Water (Yes or No) Low No High Yes Low No High Yes Claims Evidence and Reasoning Write a paragraph explaining your results. Be sure to include the following in your paragraph: What your claim was for each substance. 1. 1. Which type of bond held the atoms of each substance together. Evidence to support or refute your claim. 2. 1. Use your observations. (Both numbers and senses) Reasoning 3. 1. Explanation of what you saw happen. (Explain data and use science to explain what you know about compounds, elements, and bonding) Answers Covalent Vegetable Oil Sugar Ionic Salt Baking Soda