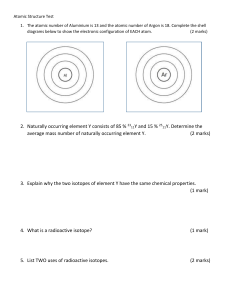
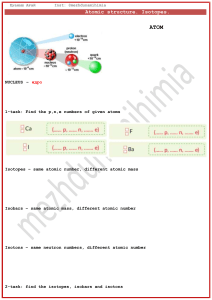
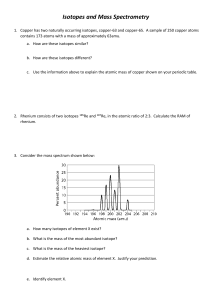
Home Learning Isotopes and Relative Atomic Mass Use the table below to calculate the relative atomic masses for each element. Element Lithium Isotopes Percentage Abudance 7.6% (6 x 7.6%) + (7 x 92.4%) Ar = 6.92 92.4% Magnesium 79% 10% (24 x79%) + (25x10%) + (26 x 11%) Ar = 24.32 11% Copper 70% (63x70%) + (65x20%) 30% Ar = 63.3